Medically reviewed by Dr. Jordan Pinsker, Chief Medical Officer, Tandem Diabetes Care.
An insulin pump is a device that can directly administer insulin to the body for someone who is living with type 1 diabetes or type 2 diabetes and requires insulin therapy. Insulin pumps are considered an alternative insulin therapy method to multiple daily injections (MDI), insulin pens, or inhaled insulin.
Why Do People Need Insulin?
Insulin is a hormone produced in the pancreas that allows the body to turn glucose into energy. A healthy pancreas is constantly producing insulin to account for the body’s energy needs. But because someone living with diabetes can’t make enough or any insulin, it must be administered to avoid severe medical complications.
Someone living with type 1 diabetes, an autoimmune disease, is unable to produce insulin or efficiently use the insulin their body produces. A person with type 2 diabetes typically has “insulin resistance,” and sometimes must supplement the insulin their body makes with insulin therapy.
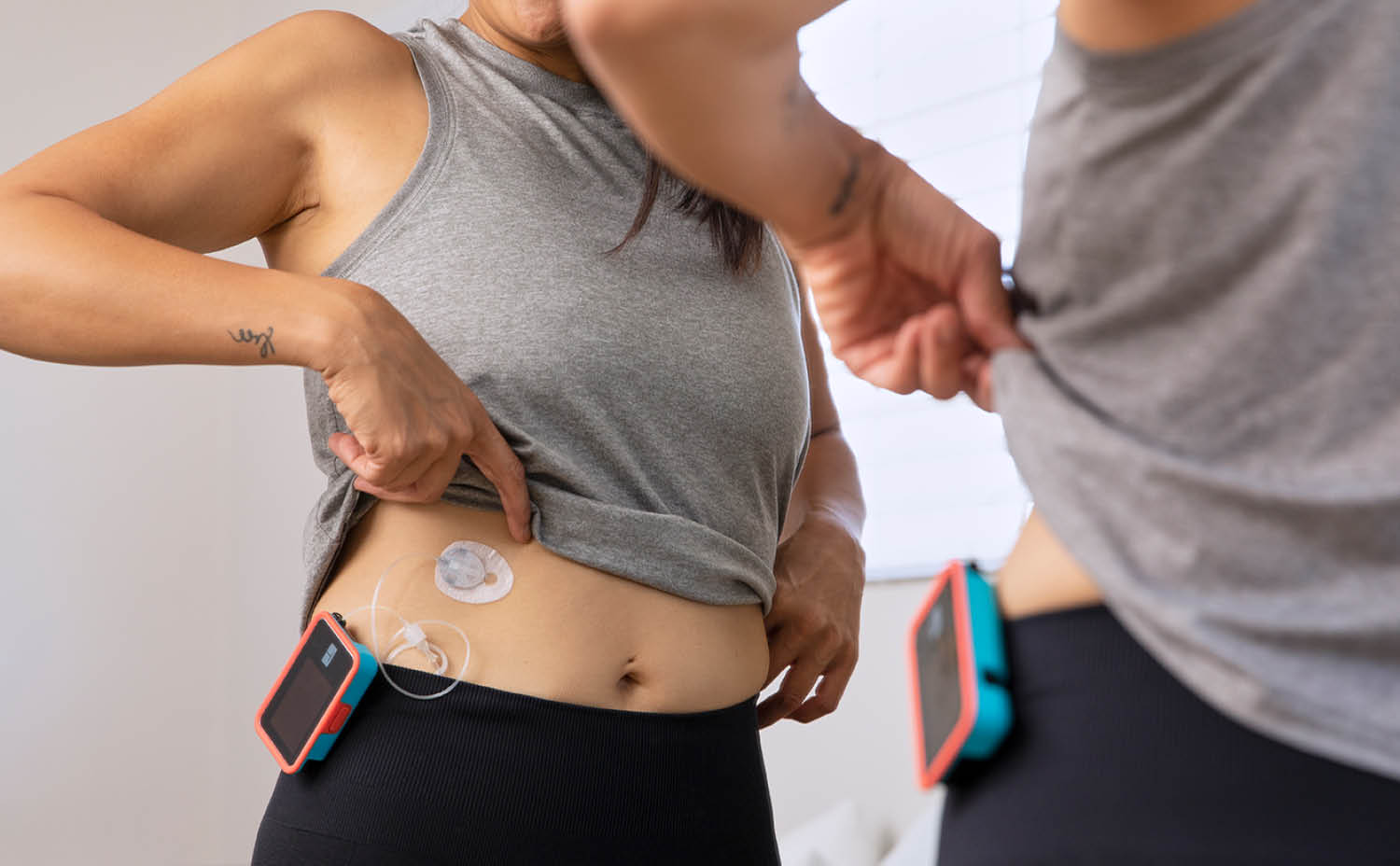
How Does an Insulin Pump Work?
Insulin pumps are connected to the body and are able to deliver insulin subcutaneously (below the skin) for someone in need of insulin therapy.
Some insulin pumps have an infusion set that connects the pump to the body through a tubing system. Others adhere directly to the body, but also have a small cannula that gets inserted through the skin.
An insulin pump holds a set amount of insulin in a cartridge or reservoir and then delivers it to the body through the skin, where it then enters the bloodstream. From there, the insulin allows cells of the body to absorb glucose and metabolize it as energy.
An insulin pump can closely mimic the body's physiological needs by slowly releasing insulin into the body throughout the day and providing a larger dose at meals.
What Are the Benefits of an Insulin Pump?
There are several benefits to using an insulin pump, including the decreased number of injections necessary to manage glucose. An integrated continuous glucose monitoring (CGM) sensor with pump therapy can also decrease the need for fingersticks.
An insulin pump can:
- Be paired with a CGM sensor
- Reduce the need to inject insulin, as most of the time the insertion set only needs to be placed once every few days (approximately every 72 hours)
- Administer basal (background) insulin throughout the day
- Administer a bolus of insulin
“Insulin pumps offer many advantages over multiple daily injections,” said Dr. Jordan Pinsker, Vice President and Medical Director for Tandem Diabetes Care and a leading pediatric endocrinologist. “Most of the time, the insertion set is left on for up to three days, making it much easier to give insulin. In addition, by infusing only rapid-acting insulin, pumps paired to sensors that support automated insulin delivery (AID) can very quickly adjust the insulin dose up or down, helping to improve time in range.”
What Types of Insulin Go in Insulin Pumps?
Insulin pumps offer two types of delivery of the same insulin. Typically, rapid-acting insulin such as Aspart (NovoLog) or Lispro (Humalog) are put into a pump. This rapid-acting insulin can be given little by little each hour to mimic the basal insulin (or background insulin) the body needs for everyday life.
“I describe the basal rate of a pump as being similar to a drip irrigation system, constantly putting out a little bit of insulin,” said Dr. Pinsker.
At mealtimes the user can bolus (give a volume or burst) insulin to match the carbohydrates in the meal they are about to eat. Users can also give correction doses. Some pumps that integrate with CGM are capable of automatically adjusting the basal rate of the pump, and some can even automatically deliver a “correction bolus” to help regulate glucose levels.
What is CGM?
A continuous glucose monitoring system attaches to the body and delivers real-time readings of glucose levels by measuring glucose in the interstitial fluid under the skin. Note that this is slightly different than direct measurements from a blood glucose meter.
“As soon as someone is diagnosed with type 1 diabetes, the standard of care should be to start on a CGM immediately,” said Dr. Pinsker, who heralds CGM technology as one of the greatest breakthroughs for diabetes management.
What Are the Different Types of Insulin Delivery Systems?
An insulin pump is just one method to deliver insulin. When starting insulin therapy, insulin is given by injections with either a syringe or insulin pen and is injected in the subcutaneous tissue just under the skin.
Insulin pens can help with more exact dosing, but they can be costly and are limited to the types of insulin they can carry.
Inhaled insulin is fast-acting, though it is not cleared for pediatric use and it can come with additional testing requirements.
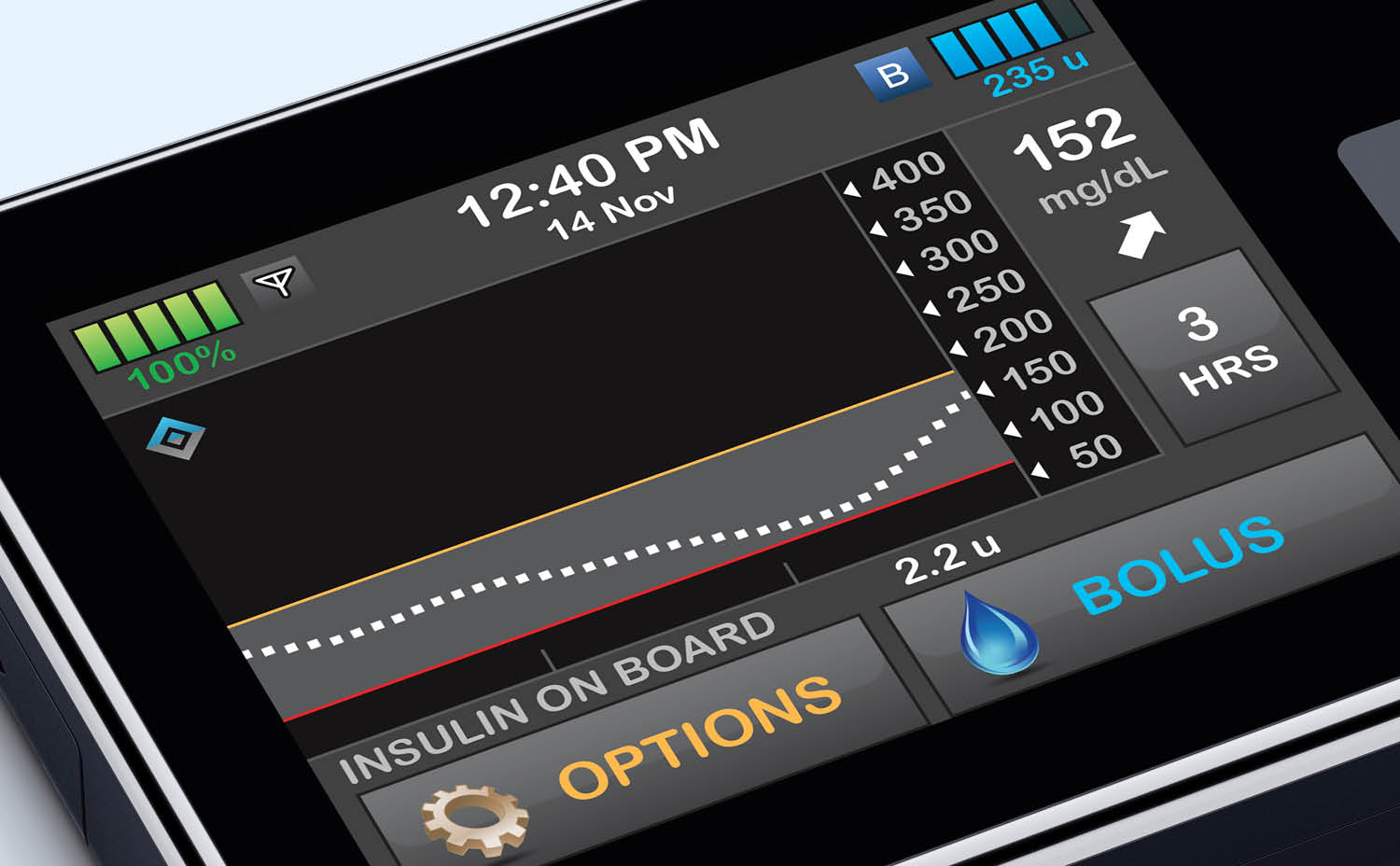
How Have Insulin Pumps Evolved?
Insulin was first discovered in 1921, and the last century has brought amazing technological advances for how diabetes is treated and insulin is administered.
The first insulin pump was invented in 1963 by Dr. Arnold Kadish. And while the first pump looked like the backpack from an astronaut's spacesuit, and though the first commercially available pump was still more than a decade away, it was proof that continuous subcutaneous insulin infusion (CSII) was possible.
Today’s insulin pumps can be very small, have Bluetooth® connectivity, and can pair with CGM sensors that allow for AID and the use of powerful, predictive algorithms that can automatically adjust insulin dosing.
“Technology has helped diabetes treatments come a long way and offers ways to help manage the disease, while reducing the burden of management on the user,” Dr. Pinsker said. “Insulin pumps that pair with a CGM offer incredible advantages that just were not possible even a few years ago.”
About Tandem Insulin Pumps
Tandem automated insulin delivery systems, which include Tandem Mobi and the t:slim X2 insulin pump, use Control-IQ technology paired with CGM to automate insulin delivery, or work as stand-alone insulin pumps. Tandem Mobi is the world’s smallest, durable automated insulin delivery system,1 while the t:slim X2 is the #1 rated insulin pump and AID system.2
Learn more about Tandem Mobi from Tandem Diabetes Care.
Learn more about the t:slim X2 insulin pump from Tandem Diabetes Care.
Unless otherwise noted, all medical information was provided by Jordan Pinsker, MD, of Tandem Diabetes Care, Inc. and Molly McElwee Malloy, RN, CDCES, formerly of Tandem Diabetes Care, Inc.
References
1. As of May, 2024. Data on file, Tandem Diabetes Care. 2. dQ&A US Patient Panel Q3 2023 (July-Sept. 2023)
Important Safety Information
RX ONLY.
Indications for Use
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater.
t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient use. The pump is indicated for use with NovoLog or Humalog U-100 insulin. The pump is indicated for use in individuals 6 years of age and greater.
Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
Warning: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of a Tandem insulin pump and Control-IQ technology must use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The Tandem pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.