Trying to keep your blood sugar in range can be stressful. Our t:slim X2 insulin pump with Control‑IQ advanced hybrid closed-loop technology offers unique features to better manage your diabetes.
Automatic Insulin Adjustments
Control-IQ technology decreases or stops basal insulin if readings from compatible continuous glucose monitoring (CGM)* sensors are predicted to be low. It increases basal insulin if sensor readings are predicted to be high.
Automatic Correction Boluses
It can also deliver automatic correction boluses (up to one an hour) to help prevent hyperglycemia.†
Activity Settings
Includes optional settings for Sleep Activity or Exercise Activity that adjust the range of treatment values.
Goodbye Fingersticks
For calibration or mealtime dosing, when the t:slim X2 insulin pump is integrated with compatible CGM.‡

Powerful Features
The beauty of the t:slim X2 pump is that it is up to 38% smaller than other leading pumps,§ yet it can hold a large amount of insulin (up to 300 units).
Color Touchscreen
Easy to read for anyone familiar with a smart device
Custom Settings
Create up to six Personal Profiles
Rechargeable Battery
Convenient charging via micro-USB port
Watertight Construction
Tested to 0.91 meters for 30 minutes (IP27 rating)
Our remote software updates provide eligible t:slim X2 pump users access to future features and predictive technology without having to purchase new device hardware.^
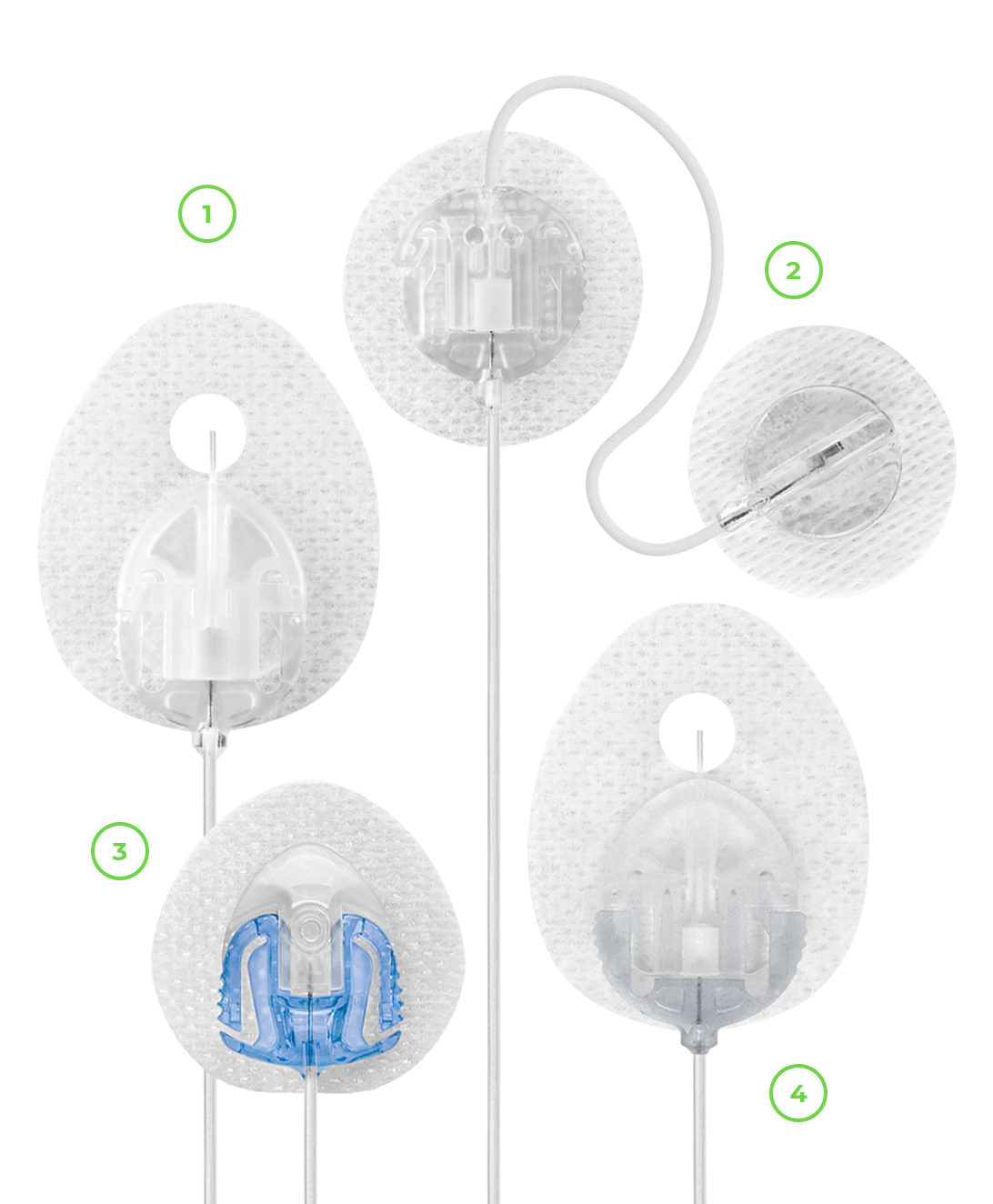
Infusion Sets
An infusion set is a complete tubing system, changed every 2-3 days, which delivers insulin from the insulin pump to your body. Finding the right infusion set is an important part of successful insulin pump therapy, which is why we offer different options.
VariSoft: Variable angle soft cannula
TruSteel: 90-degree stainless steel needle
AutoSoft 90: 90-degree soft cannula
AutoSoft 30: 30-degree soft cannula
Support Resources
* CGM sold separately.
† If glucose values are predicted to be above 10,0 mmol/L, Control-IQ technology calculates a correction bolus using the Personal Profile settings and a target of 6,1 mmol/L and delivers 60% of that value.
‡ If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
§ 38% smaller than MiniMed 780G. Data on file, Tandem Diabetes Care.
^ Additional feature updates may not be currently available for the t:slim X2 insulin pump with Control-IQ technology. Control-IQ technology is available only to customers who reside in the United States or Canada, and other select geographies. Future updates for all or some of Tandem’s products may not be developed and may not be offered everywhere and would be subject to applicable regulatory approvals. Software updates are only available to customers who are in warranty at the time they update their pump. Additional training may be required to access certain future software updates. Charges may apply. Tandem may discontinue select software and features over time at its discretion.
Important Safety Information
RX ONLY. The t:slim X2 pump and Control-IQ technology are intended for single patient use. The t:slim X2 pump and Control-IQ technology are indicated for use with NovoLog or Humalog U-100 insulin. t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is indicated for use in individuals 6 years of age and greater. Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater.
Warning: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of the t:slim X2 pump and Control-IQ technology must: use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The t:slim X2 pump must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.
These infusion sets are indicated for the subcutaneous infusion of insulin administered by the t:slim X2 insulin pump for the treatment of diabetes. These infusion sets are indicated for single use. For contraindications, warnings, precautions, and other important information, please refer to the instructions for use accompanying the infusion sets.

