Tested. Trusted. Better.
Tandem Diabetes Care automated insulin delivery systems are now powered by Control-IQ+ technology — the only predictive algorithm with AutoBolus. Control-IQ+ predicts and helps prevent highs and lows for more time in range.
Choose which Tandem automated insulin delivery system best fits your lifestyle.
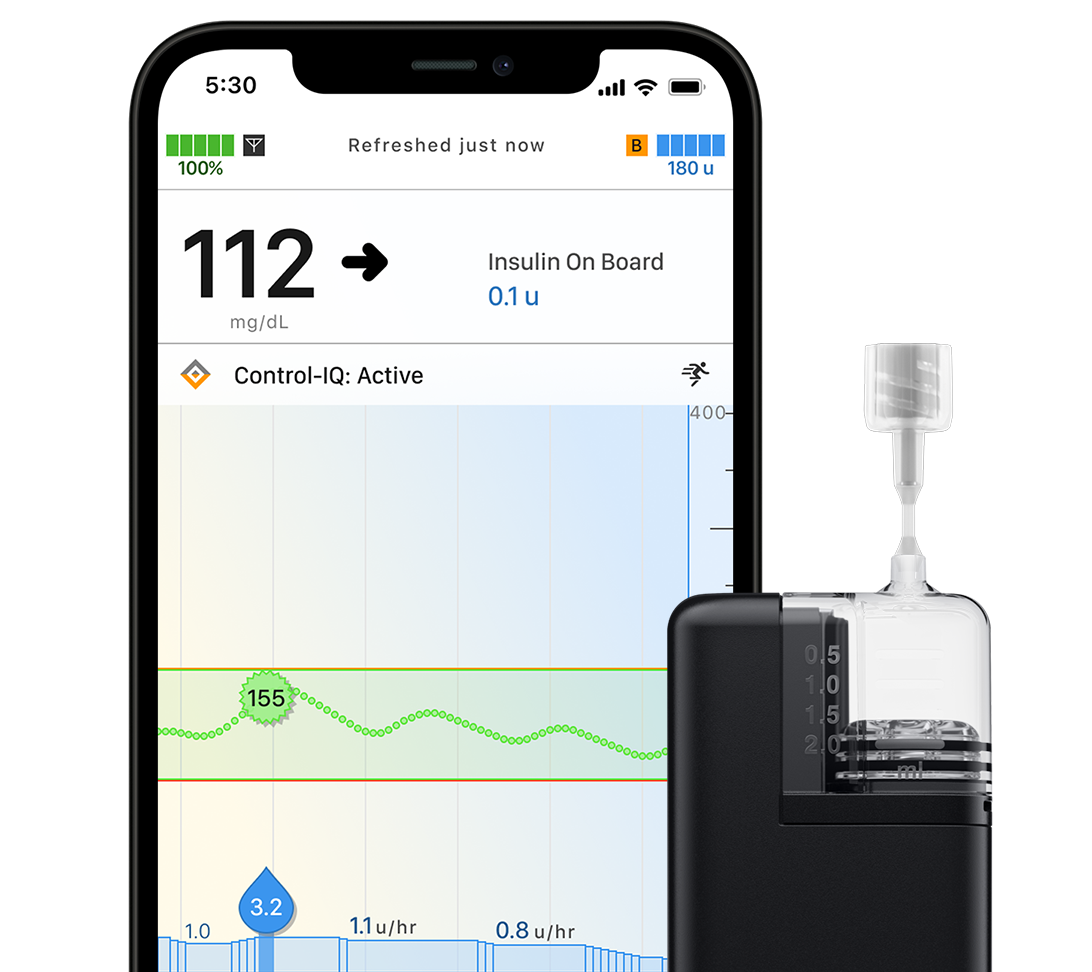
Tandem Mobi System
This insulin pump can be worn almost anywhere,* giving you more options for how you manage your diabetes. It’s powered by Control-IQ+ technology and is controllable with a compatible, personal iPhone.†
Explore Pump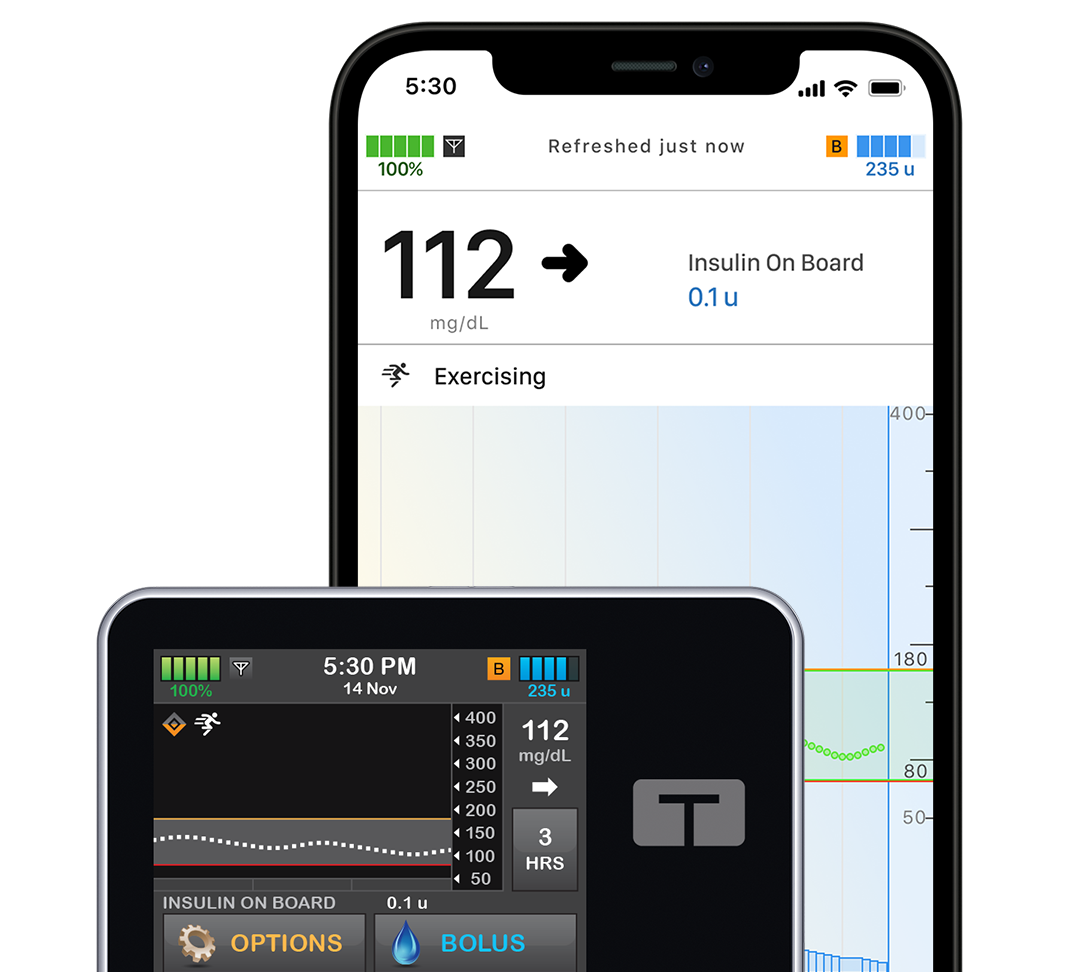
t:slim X2 Insulin Pump
This all-in-one system features a slim, sleek, user-friendly color touchscreen and multiple CGM options. It’s powered by Control-IQ+ technology, which predicts and helps prevent highs and lows.
Explore PumpBest-in-Class Outcomes Starting on Day 11
Hear from parents, kids, and teens who have experienced the life‑changing benefits of Tandem automated insulin delivery.
My A1c has been amazing since I switched to Tandem. I used to wake up with lows, and that’s really scary. But Tandem is my safety net.
Individual results may vary.
Izzy
diagnosed 2016

Tandem puts my diabetes on the backburner and lets me focus on what’s important.
Individual results may vary.
Tristan
diagnosed 1997

We sleep at night. That’s humongous. Tandem takes so much stress off of our plate.
Individual results may vary.
Kim
Mother to Anna, diagnosed 2020

If I'm performing, I don't have to worry about going low. And if I'm at church or wearing a dress, I can just go to my phone to bolus. It's so convenient.
Individual results may vary.
Eva
diagnosed 2015

Infusion Sets
Choose from a variety of cannula materials, tubing lengths, and insertion angles to fit your needs.
Software Updates
Technology evolves at a rapid pace. That’s why our insulin pumps are capable of remote software updates.
Innovative Tools
Our mobile and cloud-based applications are designed to help you better manage your diabetes.
24/7 Support
Our support team is available 24 hours/day, 365 days a year.
Expert Training
We offer personal pump training to new customers so they can quickly learn our easy-to-use products.
Limited Warranty
We’re there for you! We can replace your insulin pump to minimize any disruption to your insulin therapy.‡
What's next?
Whether you're ready to get a Tandem insulin pump, or looking to check insurance coverage, you’ll find everything you need here.

