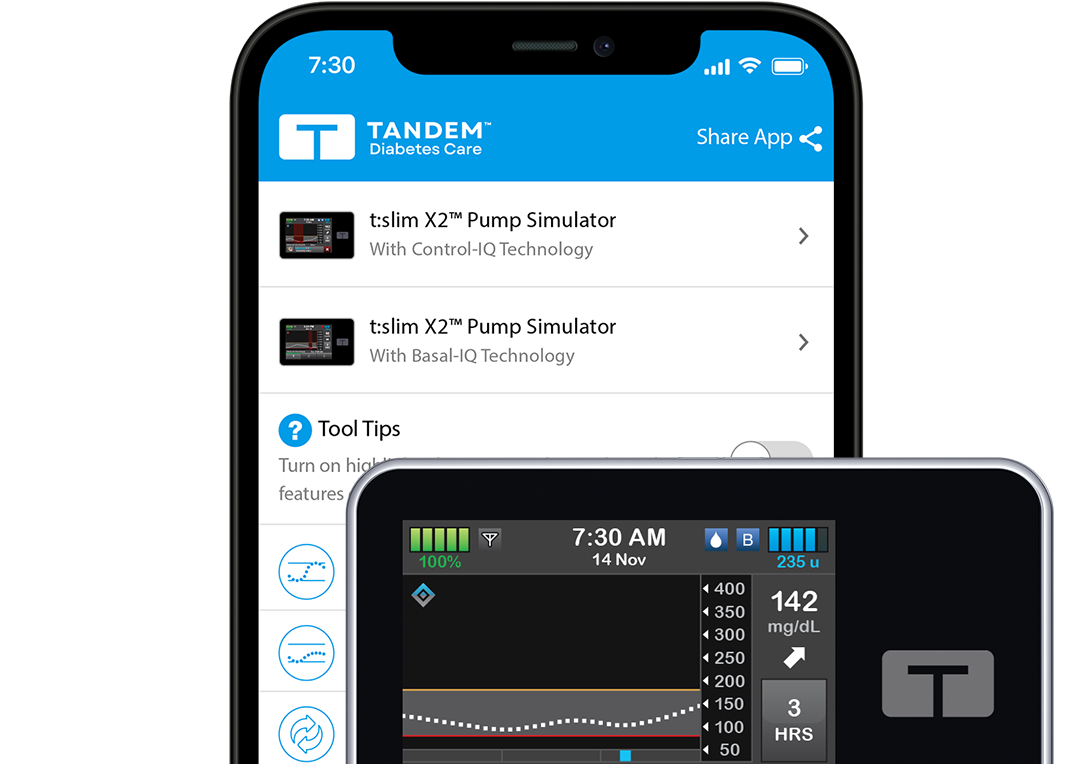
Free Virtual Demos Choose a Pump Simulator
Tandem Diabetes Care is the #1 favorite insulin pump brand.1 Test drive the easy‑to‑use interfaces of our pumps, with no obligation.
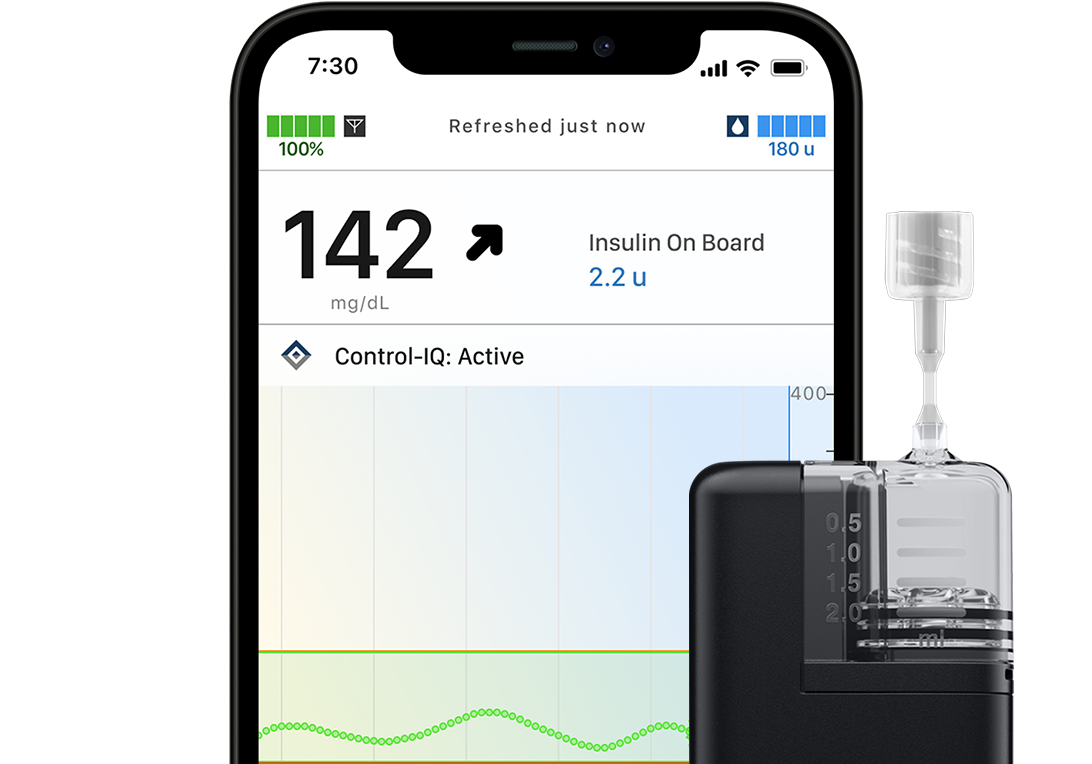
t:simulator Demo App
Experience the easy-to-use interface of the t:slim X2 insulin pump on your smartphone.
Simulator Tool Tips
Key features on the insulin pump are highlighted and explained to help you get started. You can turn Tools Tips off (or back on) at any time.
Additional Resources
Learn about pump specifications, access a glossary of common terms, or contact a Tandem Diabetes Care representative, all from within the app.

What's next?
Whether you're ready to get a Tandem insulin pump, or looking to check insurance coverage, you’ll find everything you need here.
Please Note
These applications are for demonstration purposes only. They should not be used for therapy decisions. They are intended to illustrate the user interface on a Tandem Diabetes Care insulin pump. These applications are provided on an "as-is" basis, without any warranty, and are not identical to the functionality of the pumps. These applications should not be relied upon for instruction on use of the pumps and are not a substitute for the user guides or training by a certified pump trainer.
References
1. Seagrove PWD Survey: Nov. 2024.
Important Safety Information
RX ONLY.
Indications for Use
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The Pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is indicated for use in persons 2 years of age and greater.
t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is indicated for use in persons 2 years of age and greater.
Control-IQ+ technology: Control-IQ+ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ+ technology is intended for the management of type 1 diabetes mellitus in persons 2 years of age and greater and of type 2 diabetes mellitus in persons 18 years of age and greater.
Warning: Control-IQ+ technology should not be used in anyone under the age of 2 years old with type 1 diabetes or under the age of 18 years old with type 2 diabetes. It should also not be used in patients who require less than a total daily insulin dose of 5 units of insulin per day or who weigh less than 20 pounds (9 kilograms), as those are the required minimum values needed for Control-IQ+ to operate safely.
Users of the pump and Control-IQ+ must: use the insulin pump, iCGM, and all other system components in accordance with their respective instructions for use. Failure to follow these instructions for use could result in an over delivery or under delivery of insulin. This can cause hypoglycemia (low BG) or hyperglycemia (high BG) events. Visit tandemdiabetes.com/safetyinfo for additional important safety information.