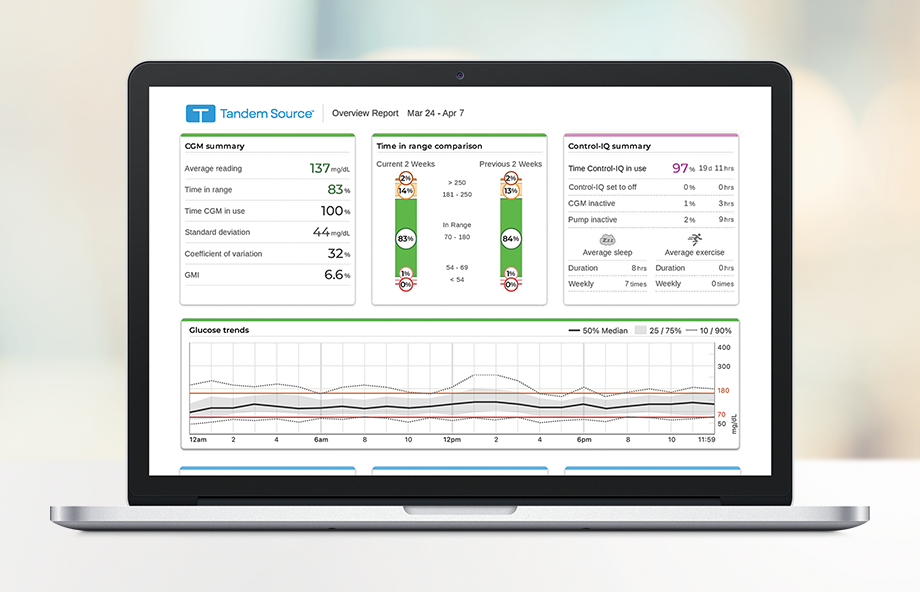
Tandem Diabetes Care automated insulin delivery (AID) systems are now powered by Control-IQ+, the only system with AutoBolus, which helps maximize outcomes and can be optimized for even more patient needs.
Tested
Tandem AID systems have been peer-reviewed in more than 115 manuscripts. There are more than 440,000 Tandem AID users worldwide with more than 300 million patient days.1
Trusted
Immediate and sustained improvements across patient populations with significantly fewer adverse events.2 Only Control-IQ+ has AutoBolus to help reduce user burden.
Better
Expanded weight and age indications for very small and very large insulin requirements with unique, optional features to quickly adjust for lifestyle and fluctuating insulin needs.
Better Management for Type 2 Diabetes
Control-IQ+ is the only automated insulin delivery (AID) system with a randomized controlled clinical trial3 studying adults with type 2 diabetes that outperforms standard insulin therapies* with a CGM.†
Learn More
Why Control-IQ+
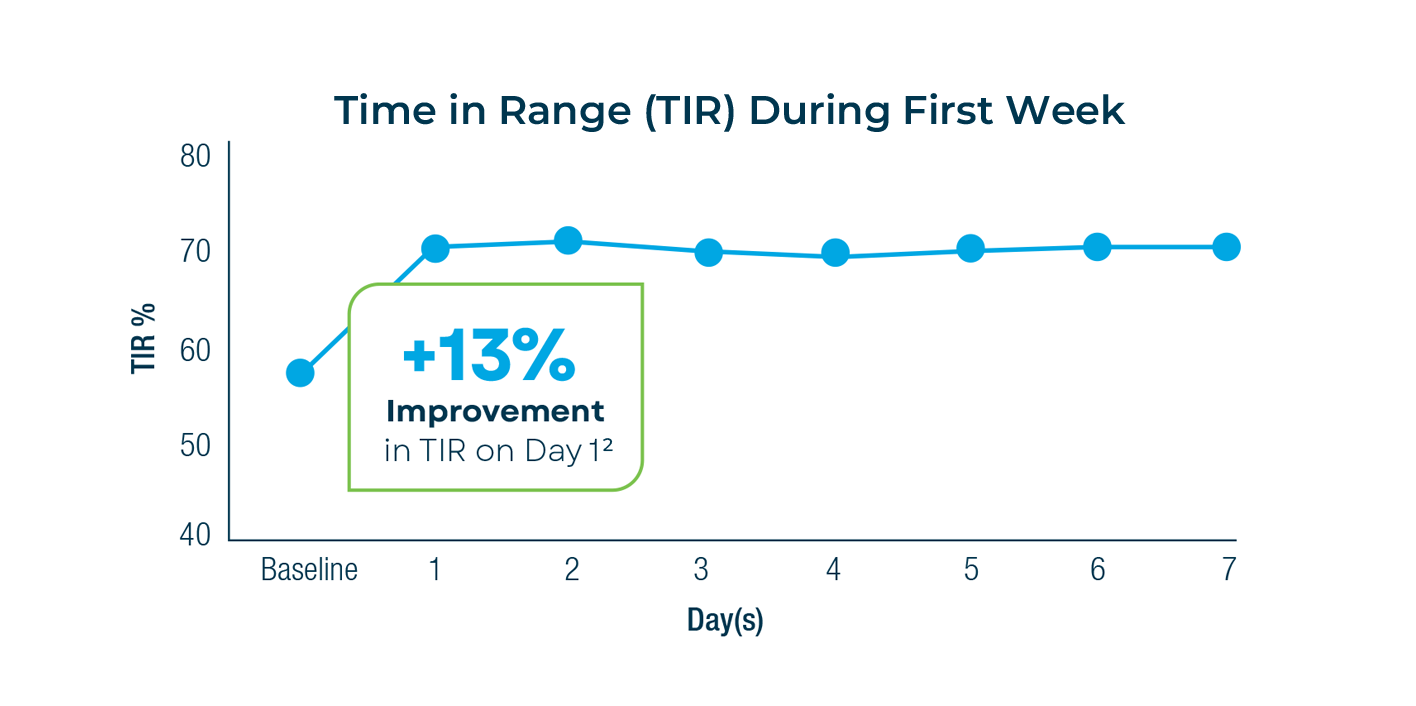
Immediate & Sustained Improvements2
Control-IQ+ has no learning time. Improvements were seen within the first 24 hours and the results were sustained during the following 13 weeks. It predicts, calculates, and automatically adjusts insulin to achieve better glucose control while providing best-in-class outcomes.
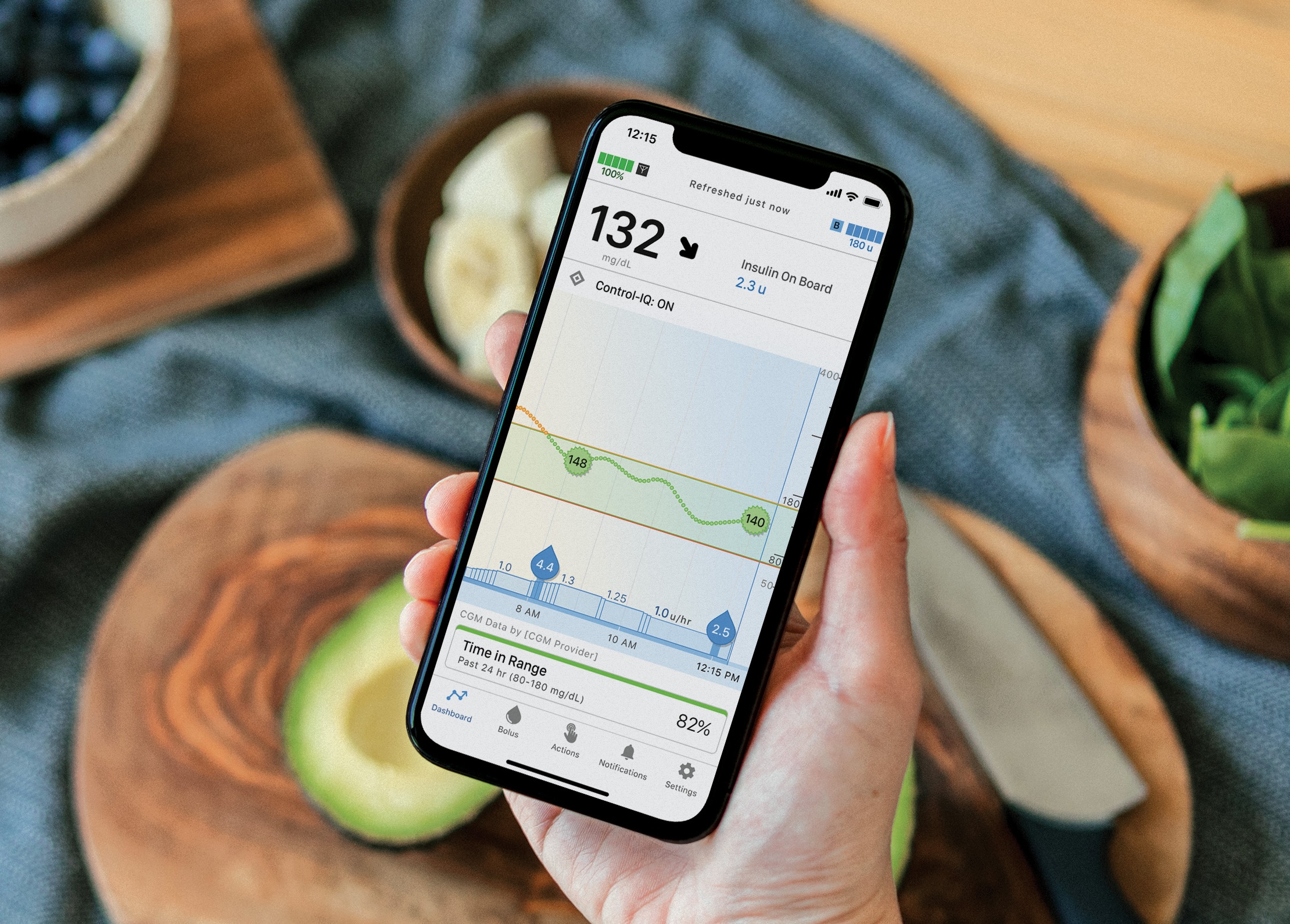
What is AutoBolus?
AutoBolus Helps Reduce Patient Burden
If sensor glucose is predicted to be above 180 mg/dL, Control-IQ+ technology will automatically deliver an AutoBolus — which is proactively delivered before glucose elevates — to help prevent hyperglycemia.‡
See How it WorksSolutions For Individual Patient Needs
Tandem Diabetes Care offers two unique AID systems, both powered by Control‑IQ+, which give your patients the options they need to best fit their lifestyle.
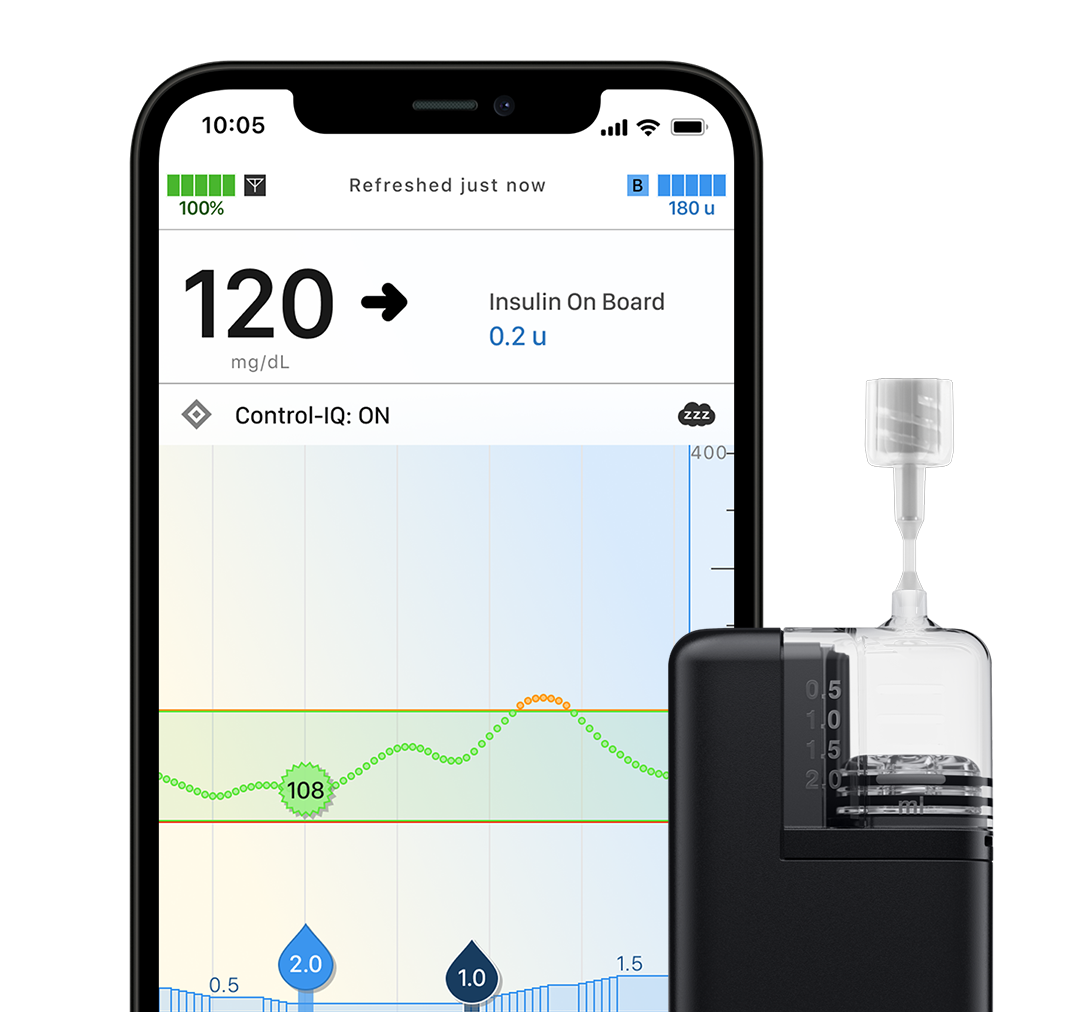
Impressively Small Tandem Mobi System
This insulin pump can be worn almost anywhere,§ giving patients more options for how they manage their diabetes. It’s also fully controllable by a personal, compatible iPhone^ with the ability to bolus from the pump if the mobile app is not available.
Explore Mobi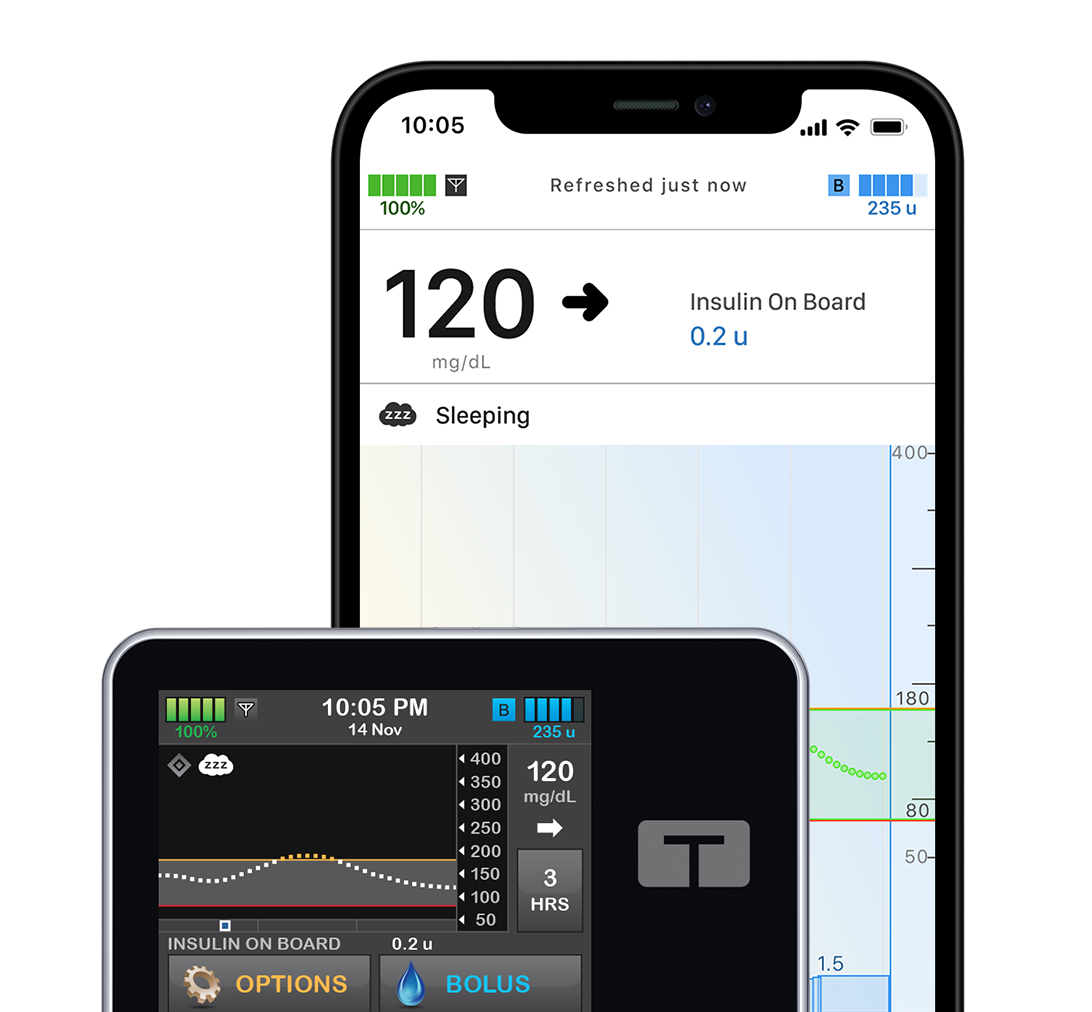
All-In-One System t:slim X2 Insulin Pump
This user-friendly, all-in-one system is sleek, modern, and has an easy-to-use color touchscreen. It pairs with multiple CGM sensors and patients can bolus from their compatible smartphone.**
Explore t:slim X2Provider Resources
Explore educational resources and product tips so your patients can make the most of their experience.
Clinical Evidence
Clinical studies have demonstrated the safety, efficacy, and ease of use of Tandem automated insulin delivery.
Industry Events
Browse live conferences, virtual events, and learning environments attended or hosted by Tandem.
Learn More Today!
Sign up to receive the latest Tandem news and educational resources.