Glycemic Outcomes by Age and Previous Insulin Delivery Method in Control-IQ Technology Users: 9 Months of CLIO Study Data
Rishi Graham1, Harsimran Singh2, Lars Mueller2, Kirstin White2, Steph Habif2, Jordan E. Pinsker2 1University of California San Diego, San Diego, 2Tandem Diabetes Care
Background
The Control-IQ Observational (CLIO) Study is currently an ongoing IRB-approved, single-arm, longitudinal study evaluating real-world use of Tandem’s t:slim X2 insulin pump with Control-IQ technology in people with T1D (PWT1D). Previous publications from CLIO have shown glycemic improvements in ethnically diverse groups of PWT1D after 3 months of using Control-IQ technology.
Method
We evaluated relationships between glycemic metrics, participants’ age, and previous insulin delivery method at 9 months after CLIO study start. Participants (N=1913) who had uploaded at least 21 days of Control-IQ feature usage data to Tandem’s t:connect web application (US only) and had ≥75% CGM use were included in the analysis. Impact of baseline factors on sensor glycemic outcomes were analyzed. Differences between baseline HbA1c and GMI were compared using a Wilcoxon test.
Results
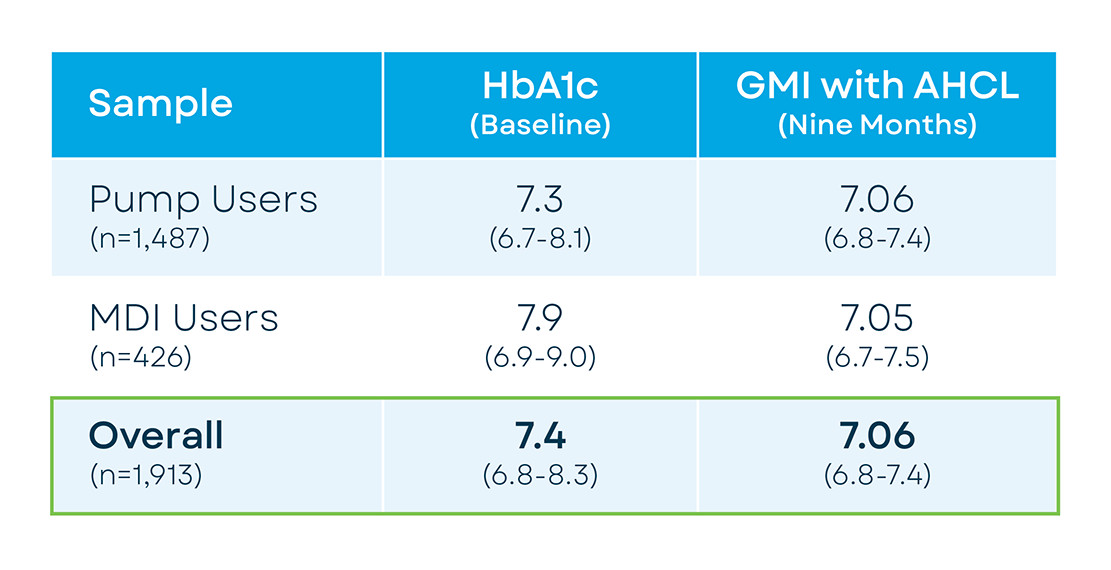
Baseline HbA1c (self-reported) for the overall sample was 7.4% (median, IQR=6.8-8.3). At post with Control-IQ technology, GMI reflected significant glycemic improvement (7.06%, IQR=6.75-7.42). Previous MDI users demonstrated 71.23% TIR (61.29-81.16) and overnight TIR=75.26% (64.62-84.5) while previous pump users showed TIR=70.43% (61.27-78.67) and overnight TIR=76.39(65.46-85.12). Participants aged ≥65 years, transitioning from prior pump therapy showed the lowest GMI (median=6.87, IQR=6.62-7.17) and the highest sensor time in range (TIR) (77.94%, IQR=69.29-83.8). Younger participants although reporting higher HbA1c at baseline (6–13-year-old, 7.8% (IQR=7.1-8.6); 14–17-year-old, 8% (IQR=7.1-9)) also showed significant glycemic improvements after 9 months of using Control-IQ technology (GMI=7.4 and 7.35, respectively).
Median Glycemic Metrics
Conclusion
Long-term use of Control-IQ technology demonstrated improved glycemic metrics irrespective of participants’ age and previous insulin delivery method.