Glycemic Outcomes for People with Type 1 and Type 2 Diabetes Using Control-IQ Technology: Real-World Data from Early Adopters
Steph Habif,* Alexandra Constantin,* Lars Mueller,† Harsimran Singh*
Objective
To compare real-world glycemic outcomes between individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) from a sample of early adopters of the t:slim X2 insulin pump with Control-IQ technology.
Method
The retrospective study included both T1D and T2D individuals who had recently updated their insulin pump software to initiate use of Control-IQ technology. Analysis included at least 14 days of pre- and 14 days of post-Control-IQ technology usage data that participants had uploaded to the t:connect web application as of March 11, 2020.
Results
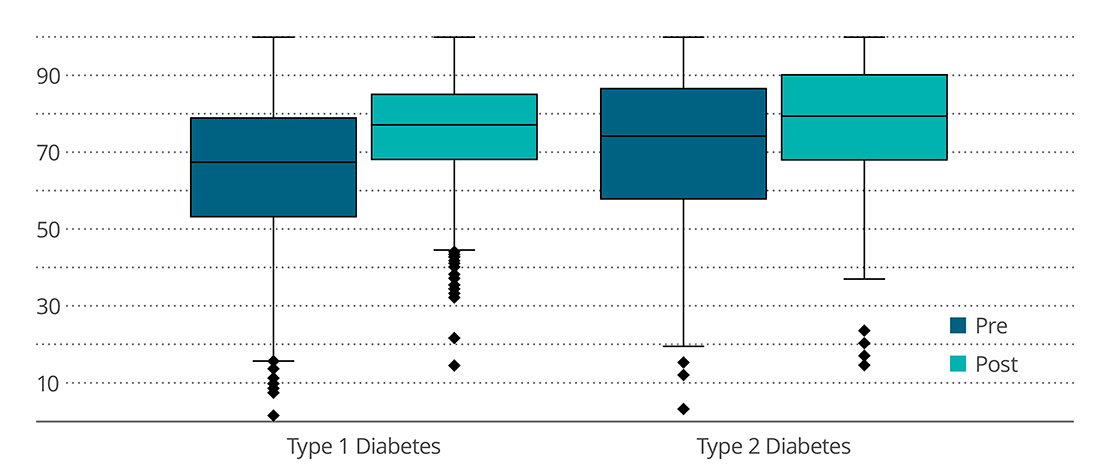
In the T1D subgroup (n=2,896), participants showed an increased sensor time in range, reduced time in sensor glucose <70 mg/dL, and reduced time in sensor glucose >180 mg/dL. In T2D subgroup (n=144), participants showed significant increase in sensor time in range and reduced time in sensor glucose >180 mg/dL. There was no change in time in sensor glucose <70 mg/dL. During 14 days of using Control-IQ technology, both T1D and T2D participants recorded 96% time in closed-loop automation.
Percentage of Time Sensor Spent in Range
Conclusion
These early results from the use of Control-IQ technology showed valuable improvements in time in range (based on sensor glucose values) and other glycemic variables for people with T1D and T2D. Improved sensor time in range, if maintained long-term, can help reduce the risk of diabetes-related complications.