Note: The post was updated 6/17/2020 to reflect an expanded pediatric indication for the t:slim X2™ insulin pump with Control-IQ™ technology to children age six and older.
It’s here! Today, we’re so excited to announce that we received FDA clearance for our Control-IQ technology for the t:slim X2 insulin pump, designed to help increase time in range, as measured by CGM, by predicting and helping prevent both highs and lows.
Control-IQ technology is the most advanced hybrid-closed loop system ever approved, and the first product approved in a new FDA category called interoperable automated glycemic controller. The FDA issuing a press release on this underscores the importance of this milestone to our industry and for people with diabetes.
What
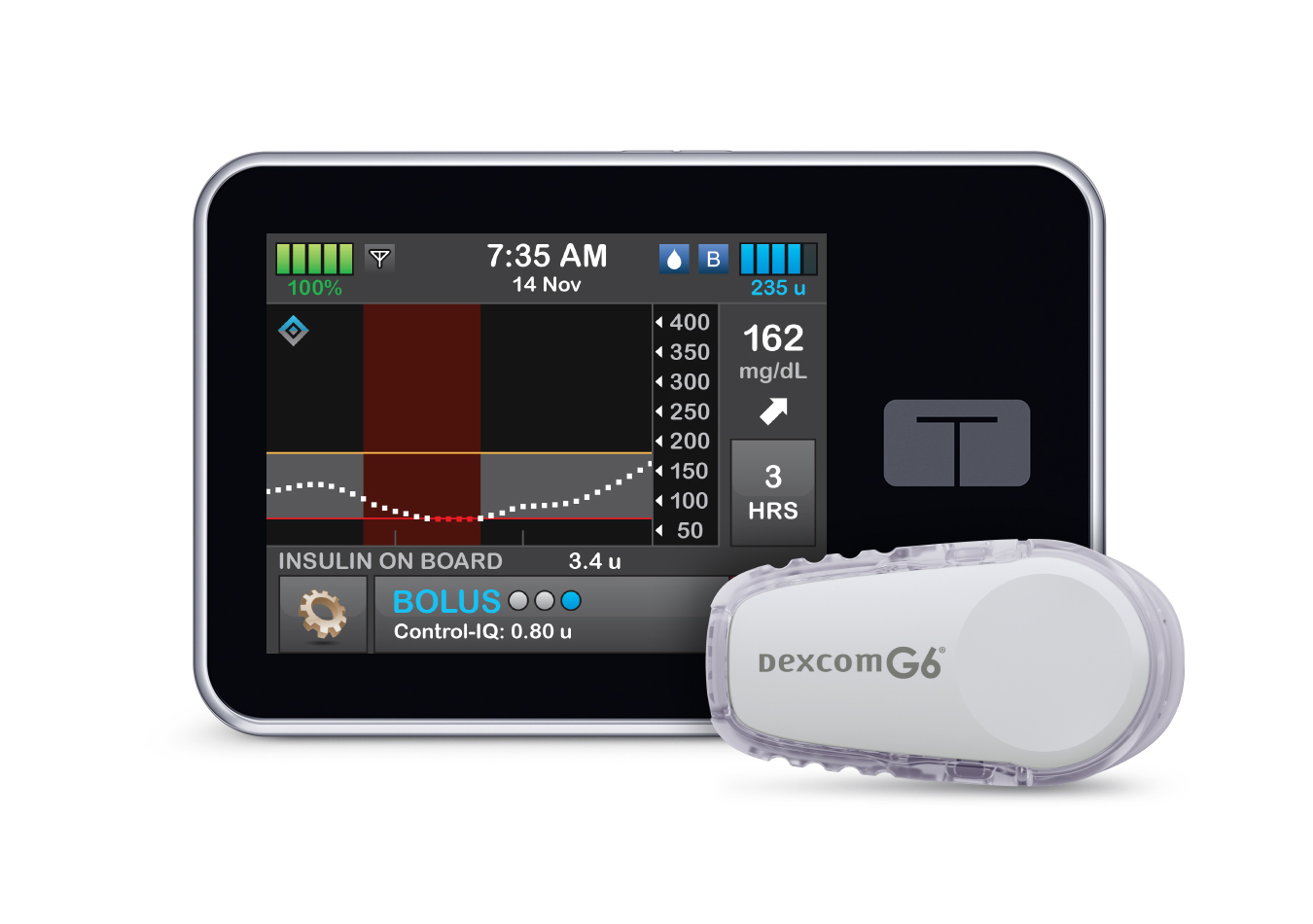
- Control-IQ technology is an advanced hybrid closed-loop system that uses Dexcom G6 CGM values to predict glucose levels 30 minutes ahead and adjusts insulin delivery accordingly, including the delivery of automatic correction boluses up to once per hour, as needed.
- If sensor glucose values are predicted to drop below 112.5 mg/dL, basal insulin delivery is reduced, and when predicted to be below 70 mg/dL, basal insulin delivery is stopped. Different treatment values may apply when a Sleep or Exercise Activity is enabled.
- If sensor glucose values are predicted to be above 160 mg/dL, basal insulin will be increased. If sensor glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates an automatic correction bolus with a target of 110 mg/dL and delivers 60% of that value. Automatic correction boluses are delivered up to once/hour as needed.
In order to understand how Control-IQ technology works and why it works the way it does, it’s important to understand the difference between target range and treatment values. Target range is described in the American Diabetes Association’s Standards of Care as the ideal glucose range for people with diabetes, and is defined as between 70mg/dL and 180 mg/dL. Treatment values are the predicted CGM values that trigger Control-IQ technology to act in order to help increase time spent in the target range, as measured by CGM.
Since rapid-acting insulin takes at least 15 minutes before it starts working, and can remain active in the body for several hours, Control-IQ technology responds to treatment values in order to adjust insulin delivery before the sensor glucose value reaches the upper or lower limits of the target range.
When
t:slim X2 pumps with Control-IQ technology are expected to begin shipping to new US customers by the end of January 2020, but any t:slim X2 pumps shipped between now and when those shipments commence can be updated to Control-IQ technology for free using the Tandem Device Updater (TDU). This update will require a new prescription and online training.
Software updates for in-warranty t:slim X2 pump users are expected to begin in January 2020. Access to the update requires a prescription and online training, which will be handled through the t:connect portal. A link to the portal and details about the
process can be found at tandemdiabetes.com/updater.
Who
The t:slim X2 insulin pump with Control-IQ technology is cleared for use in the United States. Timing of launch in additional countries is dependent on a variety of logistical and regulatory factors. Visitors from outside the United States can click the language selector in the top corner of our website to see what products are being sold in what countries and to find contact information for the local distributor. The local distributor is the best contact for information on timing and product availability.
6+
Control-IQ technology is now cleared for users age 6 and up. The FDA has required a special warning called a "boxed warning" to call attention to the potential for serious injury that could result if Control-IQ technology is used by certain patient populations, such as patients under the age of 6. For this reason, Control-IQ technology should not be used by anyone under the age of 6, or anyone who uses less than 10 units of insulin per day, or anyone who weighs less than 55 lbs. Patients should discuss treatment options with their healthcare providers.
Where
Time for a New Pump?
If you purchase a t:slim X2 insulin pump today, you’ll get Basal-IQ technology. Then you can update your pump software to Control-IQ technology using a personal computer when it’s available this January.*
Update Process for t:slim X2 Pump Users
In-warranty t:slim X2 pump users in the United States will be able to add the Control-IQ feature free of charge.
The process requires a new prescription and online training, all of which will be coordinated through a simple-to-use online portal.*
Emails with instructions on how to start the process will be sent to eligible users once the update is available.
The Control-IQ software update is only compatible with the Dexcom G6® CGM. t:slim X2 pump users who have Dexcom G5® Mobile CGM supplies they plan to use may want to postpone this update until they are ready to switch to the Dexcom G6 CGM.
A link to the portal and details about the update will be found at tandemdiabetes.com/updater when available.
* A prescription and additional training may be required to access certain future software updates. Offer only available to customers who reside in the United States, who are in warranty at the time they update their pump and who purchased a t:slim X2 insulin pump on or before December 31, 2020. Tandem may discontinue select software and features over time at its discretion. This is a limited time offer and Tandem reserves the right to discontinue this program at its discretion.
From time to time, we may pass along: suggestions, tips, or information about other Tandem Insulin Pump user experiences or approaches to the management of diabetes. However, please note individual symptoms, situations, circumstances and results may vary. Please consult your physician or qualified health care provider regarding your condition and appropriate medical treatment. Please read the Important Safety Information before using a Tandem Diabetes Care product.